Description
MycAwayTM Mycoplasma Real-time qPCR Detection Kit (2G) is a product which can qualitative detect the mycoplasma contamination in the raw materials, cell bank, virus seeds, virual or cell harvesting solution and cells used in clinical treatment, etc. The kit uses the Taqman fluorescent probe (which included FAM and CY5) and the Multiple polymerase chain reaction (PCR) tools to detect the target and internal control separately. It covered over 183 species of the Mollicutes DNA, the specificity, detection limit and robustness of this kit are validated according to EP2.6.7 which with high sensitivity, specificity, efficiency and safety. The detection limit is equals to and below 10 CFU/mL.
This product can be used in combination with Magnetic Residual DNA Sample Preparation Kit (Cat#18461ES/18469) which using the manual extracted method for the nucleic acid extraction. (Please note, the kits which included Cat#18461ES and Cat#40619ES are full validated, please contact our technical support for detailed validation information). After the samples are pre-treated to remove the interference impurities and obtain purified nucleic acid, then a qPCR reaction perform by the Real Time PCR amplifier and the fluorescence signal of the probe will be collected and analyzed.
Features
Broad Detection Range: Optimized TaqMan probes detect up to 183 mycoplasma species.
Fast & Convenient: Sample prep and testing completed in under 3 hours, versus 28 days for culture(Alternative to the standard 28-day culture test).
High Sensitivity: Detects as low as 10 CFU/mL, making it a reliable alternative to culture methods.
High Specificity: 16S rRNA-based primers and probes avoid cross-reactivity with related species such as Clostridium and Sesamum.
Safe to Use: Non-infectious positive control eliminates contamination risk.
Strong Anti-Interference: Internal control (IC) detects sample inhibition or reaction errors, reducing false negatives.
Regulatory Compliance: Validated according to the requirements of EP2.6.7, JP G3 and USP 63 pharmacopoeia, in line with the standards of international authorities.
Specifications
|
Sample Type |
media, cells, raw materials; Biopharmaceutical Purification Sample, Bulk Drug Substance Sample, Cell Cultures. |
|
Detect method |
qPCR method(Taqman fluorescent) |
|
Detect Time |
<4 hours |
|
Fluorescent probe |
FAM(Target channel);VIC(Internal channel) |
|
Pretreatment kit |
Compatible with magnetic bead-based sample pretreatment kits(Cat#18461/18469) |
|
Applicable Models |
Thermo: ABI7500, ABI QuantStudio™ 5; Roche: LC 480; Bio-Rad:CFX96 Optic Module |
|
Detection Limit(LOD) |
10 CFU/mL |
|
Covered mycoplasma |
183 mycoplasma species |
|
Validation |
Validated according to EP <2.6.7> |
Components
|
Components No. |
Name |
40619ES25 |
40619ES60 |
|
40619-A |
2×MyqPCR Reaction Buffer |
375 μL |
1.5 mL |
|
40619-B |
MyPrimer & Probe Mix |
100 μL |
400 μL |
|
40619-C* |
Internal Control (IC) |
25 μL |
100 μL |
|
40619-D** |
Positive Control (PCS) |
250 μL |
1 mL |
|
40619-E*** |
DNA Dilution buffer |
500 μL |
2×1 mL |
|
40619-F**** |
Ultrapure water |
500 μL |
2×1 mL |
[Note]: *IC: Internal control.
**PCS: Positive control solution,the concentration is 1,000 copies/µL.
***DNA Dilution buffer: used for IC dilution and the template of NTC and NCS.
****Ultrapure water: used for the preparation of qPCR Mix.
Storage
This product should be stored at -25~-15℃ for 2 years.
*Upon receipt of the kit, please check whether all components are complete and immediately store them in -25~-15℃ condition if not perform the assay immediately. Please note 40619-B should be stored away from light.
Application
Mycoplasma Detection; Mycoplasma Detection for BioProduction process media, cells, raw materials.
Figures
1. Workflow

Figure 1. Workflow of mycoplasma detection using the Mycoplasma Real-time Quantitative PCR Detection Kit
2. Specificity

Figure 2. Specificity validation.
(A). Nine common sample matrices and the kit’s DNA dilution buffer showed no mycoplasma detection in the target channel (FAM, blue). Normal FAM signal was observed only when Mycoplasma fermentans was added to a sample matrix.
(B). DNA from 14 non-Mycoplasmatales strains and 6 commonly used biopharmaceutical cell lines showed no target channel amplification. In all cases, the internal control channel (Cy5, green) amplified normally.
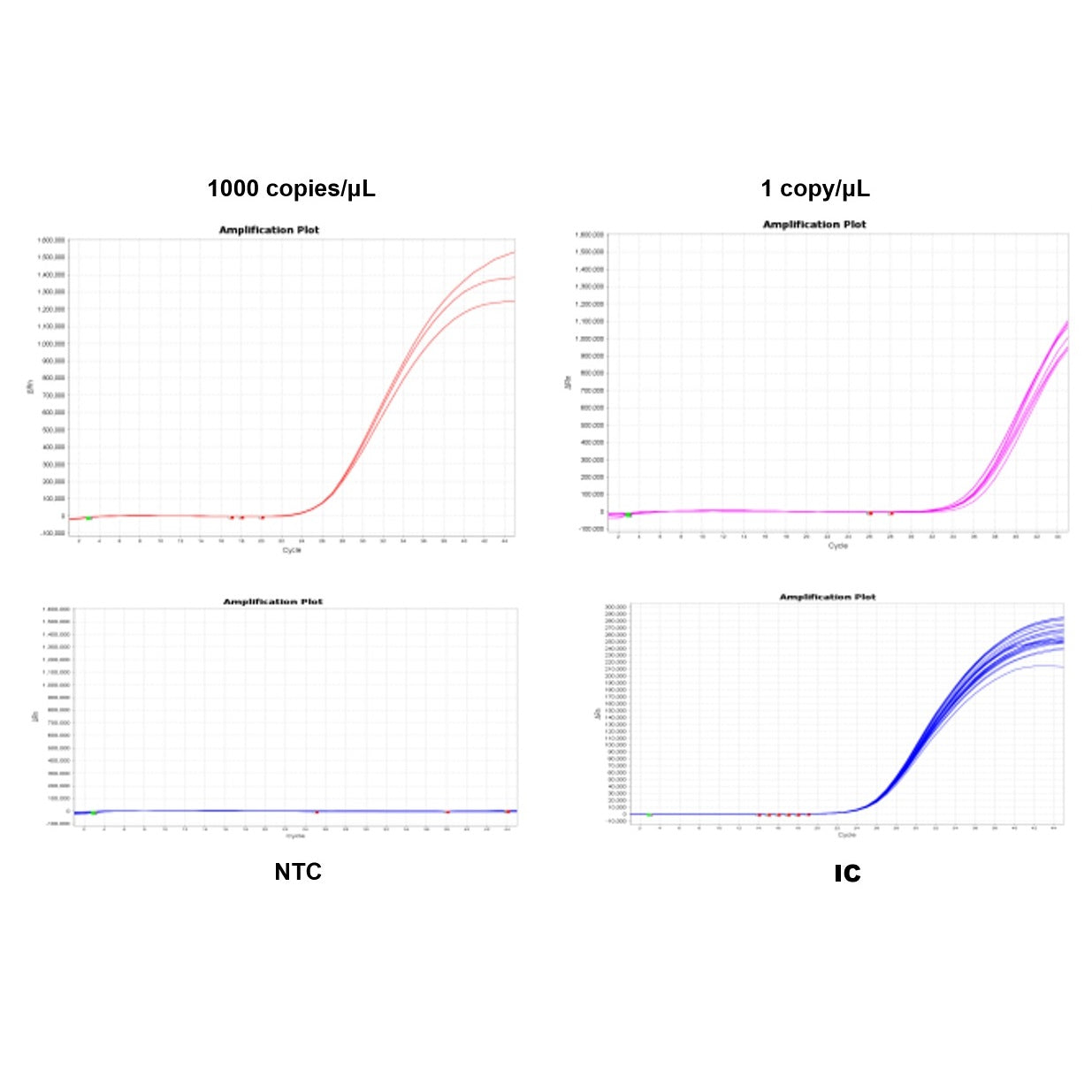
3. Limit of Detection (LOD): >95% detection rate.

Figure 3. Limit of detection (LOD) test.
Ten mycoplasma standard strains (10 CFU/mL), sourced from German MB, were tested following kit instructions for nucleic acid extraction(Cat#18461) and detection kit(Cat#40619). Mycoplasma samples were tested three times with 8 replicates each. Each run included NCS (negative control solution) and NTC (no template control).
When controls passed, at least 23 out of 24 replicates tested positive for each strain.
Documents:
Related blog:
MycAway Mycoplasma Real-time qPCR Detection Kit (2G) Validation Report
Fast, and Accurate Mycoplasma qPCR Kit
Concept of Mycoplasma and the Impact of Contamination
Mycoplasma qPCR detection and method validation contribute to the advancement of cell and gene therapy technology developmentPayment & Security
Your payment information is processed securely. We do not store credit card details nor have access to your credit card information.
Inquiry
You may also like
FAQ
The product is for research purposes only and is not intended for therapeutic or diagnostic use in humans or animals. Products and content are protected by patents, trademarks, and copyrights owned by Yeasen Biotechnology. Trademark symbols indicate the country of origin, not necessarily registration in all regions.
Certain applications may require additional third-party intellectual property rights.
Yeasen is dedicated to ethical science, believing our research should address critical questions while ensuring safety and ethical standards.