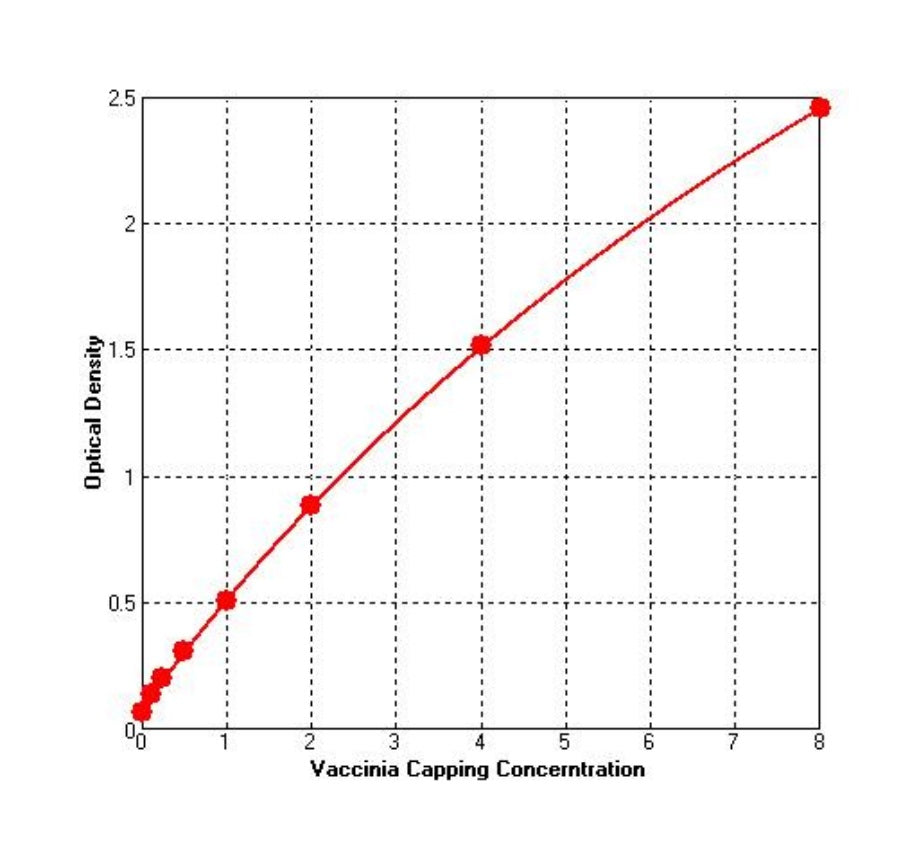
Description
In eukaryotes, mRNA is modified after transcription to form a special structure at the 5' end, i.e., the cap structure, which plays a more important role in the stabilization, transport and translation process of mRNA. Cowpox virus capsid enzyme is an effective enzyme that catalyzes the formation of the cap structure, which consists of two subunits, D1 and D12, and combines RNA triphosphate esterase activity, guanylate acyltransferase activity and guanine methyltransferase activity, which can connect the 7-methylguanine cap structure (m7Gppp) to the 5' end of RNA (m7Gppp5'N). Cowpox Virus Capping Enzyme, in the presence of appropriate concentrations of capping buffer, guanosine triphosphate (GTP), and S-adenosine methionine (SAM), is capable of capping RNAs in less than an hour and ensures proper orientation.
This kit uses the principle of double-antibody sandwich enzyme-linked immunosorbent assay (sandwich ELISA) to detect the residual Vaccinia Capping Enzyme in samples. First, add the Vaccinia Capping Enzyme standard and test sample to the Anti-Vaccinia Capping Enzyme coated microtiter strips (36709-A), then add the diluted biotin labeled Anti-Vaccinia Capping Enzyme antibody (36709-C), and finally add Streptavidin-HRP (36709-D) to form an antibody + antigen + antibody-Biotin + SA-HRP complex. Subsequently, add TMB substrate (36709-H) into the complex to observe color reaction after washing the complex. TMB is converted into blue under the catalysis of HRP enzyme and finally converted into yellow in the presence of acid, and the shade of color is positively correlated with the amount of Vaccinia Capping Enzyme in the sample.
The detection range of this kit is 0.125~8 ng/mL; the lower detection limit is 0.065 ng/mL.
Specifications
|
Cat.No. |
36709ES48 / 36709ES96 |
|
Size |
48 T / 96 T |
Components
|
Components No. |
Name |
36709ES48 |
36709ES96 |
|
36709-A |
Anti-Vaccinia Capping Enzyme coated microtiter strips |
48 T |
96 T |
|
36709-B* |
Standard: Vaccinia Capping Enzyme |
1 vial |
2 vial |
|
36709-C |
Detection Antibody:Biotin-conjugated Antibodies |
60 μL |
120 μL |
|
36709-D |
Streptavidin-HRP |
20 μL |
40 μL |
|
36709-E |
Dilution Buffer 1 |
25 mL |
45 mL |
|
36709-F |
Wash Buffer Concentrate (20×) |
25 mL |
50 mL |
|
36709-G |
Dilution Buffer 2 |
15 mL |
30 mL |
|
36709-H |
TMB Substrate |
8 mL |
15 mL |
|
36709-I |
Stop Solution |
5 mL |
10 mL |
|
36709-J |
Plate Sealer |
3 each |
5 each |
*The Standard (36709-B) is Lyophilized powder.
Storage
This product* should be stored at 2°C ~8°C. Unopened product is valid for one year.
*Upon receipt of the kit, please check whether all components are complete and immediately store them in corresponding condition.
Notes
1. Please read this manual carefully before using this kit. The kit needs to be used up within its shelf life.
2. Mixing of related reagents from different batches is prohibited.
3. The experiment should be conducted in a standardized manner, vortex and mix well for each reagents and samples before use.
4. The kit is designed for detecting the target antigens and samples marked in the instructions only. Other applications need to be designed and verified by the user, and the reliability and accuracy should be evaluated based on the results.
5. For your safety and health, please wear personal protective equipment (PPE), such as laboratory coats and disposable gloves, when operating with this product.
6. This product is for research use only.
Document:
Payment & Security
Your payment information is processed securely. We do not store credit card details nor have access to your credit card information.
Inquiry
You may also like
FAQ
The product is for research purposes only and is not intended for therapeutic or diagnostic use in humans or animals. Products and content are protected by patents, trademarks, and copyrights owned by Yeasen Biotechnology. Trademark symbols indicate the country of origin, not necessarily registration in all regions.
Certain applications may require additional third-party intellectual property rights.
Yeasen is dedicated to ethical science, believing our research should address critical questions while ensuring safety and ethical standards.