Description
T7 RNA polymerase uses double-stranded DNA containing the T7 promoter sequence (5’-TAATACGACTCACTATAG*-3’) as a template and NTPs as substrates to synthesize RNA complementary to the reverse strand of DNA downstream of the promoter. Both linear double-stranded DNA with blunt ends or 5’ overhangs can serve as template substrates for T7 RNA polymerase, meaning linearized plasmids or PCR products can be used as templates for in vitro RNA synthesis.
This kit employs a sandwich enzyme-linked immunosorbent assay (ELISA) principle to detect residual T7 RNA polymerase. T7 RNA polymerase standards and test samples are added to microplate wells pre-coated with anti-T7 RNA polymerase antibodies (36705-A). Diluted biotin-labeled T7 RNA polymerase detection antibodies (36705-C) are then added, followed by Streptavidin-HRP (SA-HRP) (36705-D), forming an antibody + antigen + antibody-biotin + SA-HRP complex. After washing, TMB substrate (36705-H) is added for color development. TMB is catalyzed by HRP to transition from colorless to blue and finally to yellow upon addition of stop solution (36705-I). The intensity of the yellow color is positively correlated with the amount of T7 RNA polymerase detected in the sample.
Features
High Sensitivity: Quantitative lower limit (LOQ) of 1 ng/mL; detection limit (LOD) as low as 0.318 ng/mL.
High Precision: Intra-assay CV <10%; intermediate precision (inter-assay CV) <15%.
High Accuracy: Spike recovery rates consistently within 70–130%.
Strong Specificity: No significant variation observed in T7 RNA polymerase values after adding various interfering substances.
Excellent Stability: Stable for 7 days at 37 °C without performance loss.
Regulatory Compliance: Fully validated in accordance with ChP, USP, and ICH Q2 (R2) guidelines.
Quality Assurance: All critical raw materials, including coated and detection antibodies and standards, are independently developed and fully traceable.
Audit-Ready: Stable manufacturing process with controlled lot-to-lot variation and comprehensive audit documentation.
Specifications
|
Parameter |
Specification |
|
Detection Range |
1 – 32 ng/mL |
|
Assay Method |
Double-antibody sandwich |
|
Assay Time |
3.5 hours |
|
Sensitivity |
0.318 ng/mL |
|
Dilution Linearity |
Difference <20% compared with undiluted samples |
|
Recovery Rate |
70% – 130% |
|
Intra-assay Variation |
<10% |
|
Inter-assay Variation |
<15% |
Components
|
Components No. |
Name |
36705S48 (48 T) |
36705ES96 (96 T) |
|
36705-A |
Anti-T7 RNA Polymerase coated microtiter strips |
48 T |
96 T |
|
36705-B* |
Standard: T7 RNA Polymerase |
1 vial |
2 vial |
|
36705-C |
Detection Antibody:Biotin-conjugated Antibodies |
60 μL |
120 μL |
|
36705-D |
Streptavidin-HRP |
30 μL |
60 μL |
|
36705-E |
Dilution Buffer 1 |
25 mL |
45 mL |
|
36705-F |
Wash Buffer Concentrate (20×) |
25 mL |
50 mL |
|
36705-G |
Dilution Buffer 2 |
15 mL |
30 mL |
|
36705-H |
TMB Substrate |
8 mL |
15 mL |
|
36705-I |
Stop Solution |
5 mL |
10 mL |
|
36705-J |
Plate Sealer |
3 each |
5 each |
Storage
Store at 2-8°C. The shelf life is 1 year when unopened, and 6 months after opening.
Upon receipt of the product, please check if all components are complete, and immediately store them at the corresponding storage temperature.
Figures
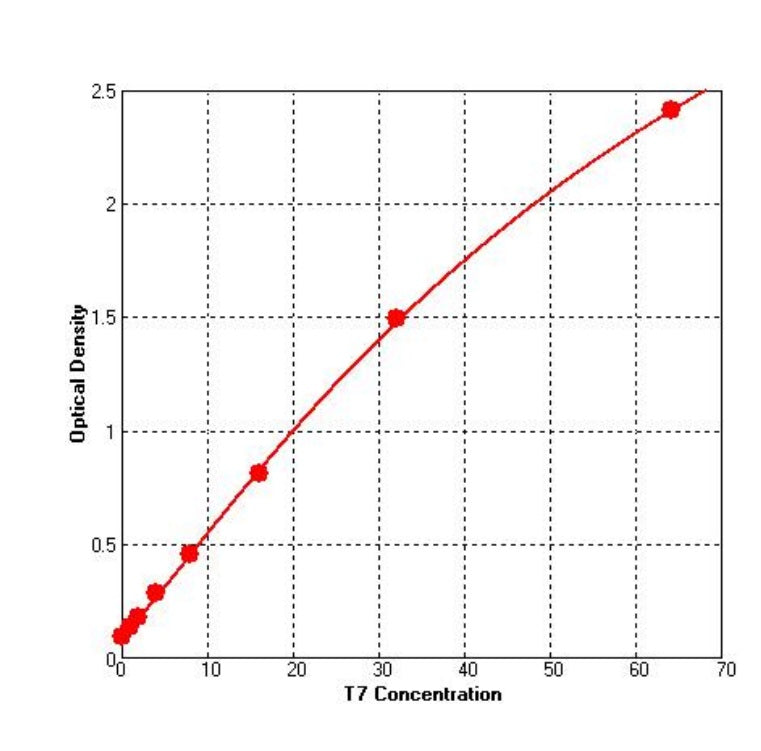
1. Good Linearity
Table 1. Linearity Range: 1~32 ng/mL,R2=0.999 ,CV≤5%。
|
Std |
Concn.(ng/mL) |
AVG(ng/mL) |
Recovery(%) |
CV% |
|
Std 1 |
32 |
31.965 |
99.9% |
2.2% |
|
Std 2 |
16 |
16.070 |
100.5% |
5.0% |
|
Std 3 |
8 |
7.812 |
97.7% |
2.1% |
|
Std 4 |
4 |
4.192 |
104.8% |
0.8% |
|
Std 5 |
2 |
2.020 |
101.8% |
3.5% |
|
Std 6 |
1 |
1.173 |
117.3% |
0.0% |
|
NC |
0 |
/ |
/ |
/ |

Figure 1. Standard curve ) for .T7 RNA Polymerase ELISA kit
2. High Specificity

Figure 2. Cross-reactivity comparison.
Eight process-related proteins were tested using the Cat#36705ES kit to evaluate potential cross-reactivity of the T7 RNA Polymerase antibody with nine different proteins. Positive control (PC, 1 ng/mL) and negative control (NC, 0 ng/mL) were included. Results showed that the eight process-related proteins (10 µg/mL) produced signals comparable to the negative control, with detected concentrations below the kit’s quantitation limit (1 ng/mL), indicating no cross-reactivity.
Table 2. Cross-reactivity Data with Proteins Added in Eight Manufacturing Processes
|
Protein Types |
OD Value |
Concentration(ng/mL) |
|
PC-1 ng/mL |
0.165 |
1 |
|
0 |
||
|
UltraNuclease |
n.d. |
|
|
Salt Active UltraNuclease |
n.d. |
|
|
DNase I |
n.d. |
|
|
Murine RNase Inhibitor |
n.d. |
|
|
Vaccinia Capping Enzyme |
n.d. |
|
|
Inorganic Pyrophosphatase |
n.d. |
|
|
mRNA Cap 2´-O-Methyltransferase |
n.d. |
|
|
1%BSA |
n.d. |
3.High Accuracy: Spike recovery rates consistently within 70–130%.
Table 3. Dilution Linearity and Spike Recovery
|
Sample Dilution |
Measured Value (ng/mL) |
Linearity (%) |
Sample + Spike |
Measured Value (ng/mL) |
Recovery (%) |
|
S + Std1 |
31.467 |
/ |
S + Std1 |
31.467 |
97.0% |
|
2× |
13.729 |
87.3% |
S + Std3 |
7.625 |
90.2% |
|
4× |
6.015 |
87.6% |
S + Std5 |
2.248 |
91.6% |
|
8× |
3.068 |
102.0% |
S (Sample) |
0.907 |
/ |
|
16× |
1.742 |
113.6% |
/ |
/ |
/ |
Equal volumes of high, medium, and low concentrations of T7 standards were spiked into mRNA purification samples, with the high-level spiked sample further subjected to serial dilutions. T7 detection results from diluted samples, spiked samples, and unspiked controls all showed linearity and spike recovery rates within 80%–120%.
Spike Recovery Rate = Measured Value / (50% Spiked Standard + 50% Sample Value)
4. High Sensitivity: Quantitative lower limit (LOQ) of 1 ng/mL; detection limit (LOD) as low as 0.318 ng/mL.
Table 4. LOQ
|
Std6 theoretical value(ng/mL) |
Std 6 test mean value(ng/mL) |
Test repetitions |
CV% |
Recovery |
|
1 |
0.858 |
24 |
12.6% |
85.8% |
The limit of quantification for this product is determined by diluting the lowest concentration point of the standard curve, where the coefficient of variation (CV) at the lowest concentration is less than 20%. This is defined as the limit of quantification, which is 1 ng/mL.
Table 4. LOD
|
Mean value(OD) |
SD(OD) |
Mean value +2SD (OD) |
Mean value +2SD(ng/mL) |
|
0.0527 |
0.0038 |
0.0604 |
0.3176 |
The detection limit of this product is defined as the standard concentration corresponding to the mean detection value of the negative control (NC) plus two times the standard deviation. By measuring 24 NC standards, calculating the mean and standard deviation, the detection limit is determined to be 0.318 ng/mL.
Documents:
Safety Data Sheet
Manuals
Payment & Security
Your payment information is processed securely. We do not store credit card details nor have access to your credit card information.
Inquiry
You may also like
FAQ
The product is for research purposes only and is not intended for therapeutic or diagnostic use in humans or animals. Products and content are protected by patents, trademarks, and copyrights owned by Yeasen Biotechnology. Trademark symbols indicate the country of origin, not necessarily registration in all regions.
Certain applications may require additional third-party intellectual property rights.
Yeasen is dedicated to ethical science, believing our research should address critical questions while ensuring safety and ethical standards.