Description
LZCap™AG(3'Ma-Cy7) is a Cap1 analog with a Cy7 fluorescent marker, which can be used as an mRNA co-transcription capping reagent. Under the action of T7 polymerase, LZCap®AG(3'Ma-Cy7), NTPs and template DNA are co-transcribed to produce mRNA with a 5'-end Cap 1 structure. The capped mRNA can be directly translated and expressed in cells and in vivo, with excellent expression efficiency. The mRNA capped by LZCap®AG(3'Ma-Cy7) can be detected by flow cytometry and fluorescence microscopy for Cy7 fluorescence, and the distribution of mRNA and LNP can be traced and located. The recommended wavelength of Cy7 fluorescence of LZCap®AG(3'Ma-Cy7) mRNA is: (651/780).
Specifications
|
Cat.No. |
10689ES10/10689ES60 |
|
Size |
50 μL、100 μL |
|
Molecular Formula |
C68H86N18O30P4S2(Free acid) |
|
Molecular Weight |
1823.55(Free acid) |
|
Concentration |
25 mM |
|
Purity |
HPLC ≥90% |
|
Salt type |
NH4+ |
|
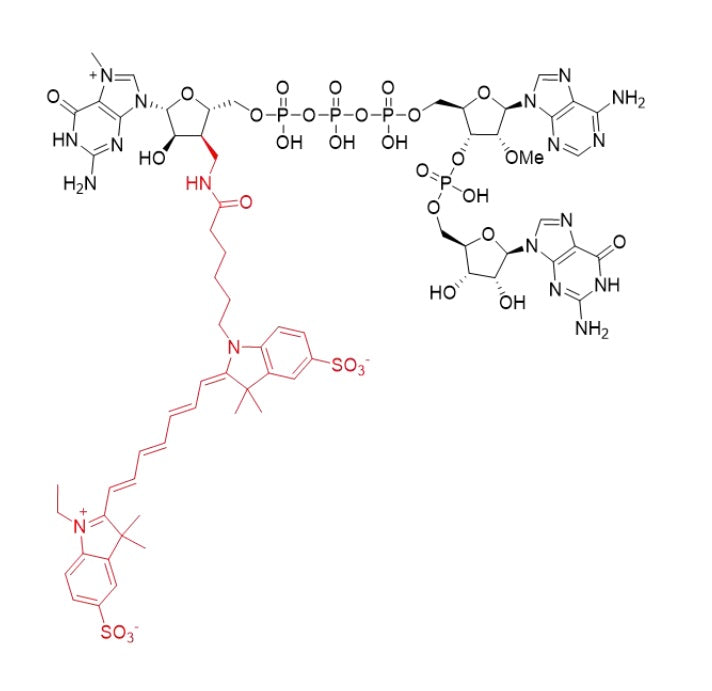
Structure |
|
Components
|
Name |
10689ES10 |
10689ES60 |
|
LZCap™AG(3'Ma-Cy7) |
50 μL |
100 μL |
Storage
This product should be stored at -25~-15℃ for 2 years
Instructions
DNA template design of LZCap™
LZCap™AG(3'Ma-Cy7) is suitable for sequences starting with AG. The T7 promoter (underlined) followed by the AG sequence can effectively initiate transcription.

Operating Instructions
1.Thaw the components needed for the experiment on ice.
2. Prepare the transcription system at room temperature according to the following reaction system.
|
Component |
Volume(μL) |
Final concentration |
|
RNase Free Water |
Make up to 20 μL |
/ |
|
ATP(100mM) |
1 |
5mM |
|
UTP(100mM) |
1 |
5mM |
|
CTP(100mM) |
1 |
5mM |
|
GTP(100mM) |
1 |
5mM |
|
LZCap®AG(3'Ma-Cy7) (25mM) |
3.2 |
4mM |
|
10×Transcription Buffer |
2 |
1× |
|
Linear DNA |
1μg |
50ng/μL |
|
Recombinant RNase Inhibitor(40U/μL) |
0.5 |
1U/μL |
|
Pyrophosphatase(0.1U/μL) |
0.4 |
0.002U/μL |
|
T7 RNA polymers(250U/μL) |
0.64 |
8U/μL |
|
Final volume |
20μL |
|
【Note】: *Modified N-Me-pUTP can be used in place of wild-type UTP.The modified N-Me-pUTP reduces theimmunogenicity of mRNA. 3. Mix the prepared reaction solution, centrifuge briefly, and incubate at 37℃ for 2-3 h. If the transcript length is less than 100 nt,increase the reaction time to 4-8 h.
3. Mix the prepared reaction solution, centrifuge briefly, and incubate at 37℃ for 2-3 hours. If the transcript length is less than 100nt, increase the reaction time to 4-8 h.
Notes:
1) LZCap™AG (3'Ma-Cy7) is suitable for T7 promoter transcription vectors with 5'AG 3' start sequence, which needs to be considered when constructing the vector.
2) LZCap™AG (3'Ma-Cy7) and its mRNA products should be stored and used away from light.
3) The reagents, consumables and containers used in the experiment are free of RNase contamination.
4) It is recommended to use linearized DNA templates for transcription.
5) When using modified nucleotides instead of wild-type nucleotides, the final concentration of the reaction remains unchanged.
6) If PCR products are used as transcription start templates, the amount of DNA template can be reduced by half.
7) Since the concentration of 10×Transcription Buffer is high, the high salt environment will cause the polymerase to be inactivated. At the same time, the buffer contains components that will form a precipitate with the template DNA. When preparing the reaction solution, it is necessary to adjust the order of adding components and calculate the system. First add water, then add buffer, NTP, and finally add template and enzyme to prevent the high concentration of 10× salt ions from affecting the enzyme.
8) In the purification of mRNA after transcription, wash the mRNA precipitate once with pre-cooled 75% ethanol. Repeated washing will affect the fluorescent group.
Documents:
Safety Data Sheet
Manuals
Payment & Security
Your payment information is processed securely. We do not store credit card details nor have access to your credit card information.
Inquiry
You may also like
FAQ
The product is for research purposes only and is not intended for therapeutic or diagnostic use in humans or animals. Products and content are protected by patents, trademarks, and copyrights owned by Yeasen Biotechnology. Trademark symbols indicate the country of origin, not necessarily registration in all regions.
Certain applications may require additional third-party intellectual property rights.
Yeasen is dedicated to ethical science, believing our research should address critical questions while ensuring safety and ethical standards.